The determination of oxygen (O2) is important in many processes. Methods for measuring oxygen contents are either physical or chemical. Physical methods use the paramagnetic property of oxygen or thermal conductivity as the basis for quantitative determinations.
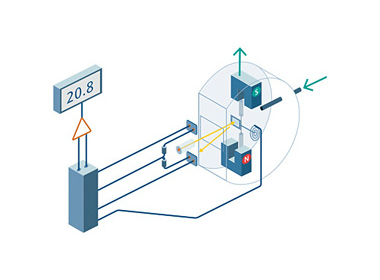
Most gases are slightly diamagnetic and repelled out of a magnetic field. Oxygen is different, it is a paramagnetic gas, which means that it is attracted by a magnetic field. There are a number of oxygen analyzers which use the unique paramagnetic properties of oxygen.
Paramagnetism is strongly temperature-dependent. Cold oxygen molecules are attracted by a strong magnetic field and when they are heated they leave the magnetic field. This gives rise to a current which generates a measure of the oxygen content.